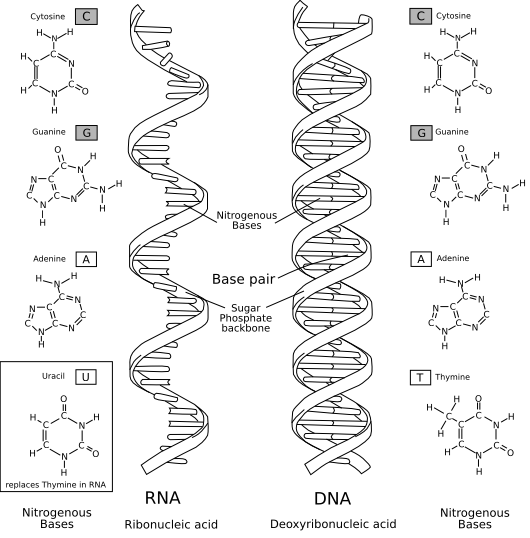
One of the 20 biggest questions in science that I have is how to beat bacteria. I am interested in this because the discovery of antibiotics greatly impacted the quality and length of human life. Now,we are being threatened by antibiotic resistant bacteria. This threatens to bring us back to times where millions of people died of bacteria causing infections every year. To combat this, people are exploring the genetic sequence of bacteria and making new antibiotics. In addition, there has been research of transplanting bacteria from fetal matter that are good to patients who have the bad bacteria.
List of Questions in Science that I have
1.) How did bacteria evolve into humans?
2.) How advanced will the Human race become?
3.) Will we ever lived in a society with artificial intelligence?
4.) Can we stop global warming?
5.) Will we be able to manipulate what genes we pass on to our children?
6.) Can we find an effective cure for viral infections?
7.) Can we make medicine with minimal side effects?
8.) Can we travel to other galaxies?
9.) Can we find a source of energy that is cheap, reliable, renewable, and non-polluting?
10.) Can we have vaccines against cancer?
11.) Can we map an evolutionary tree that maps relationships between all species that have ever existed.
12.) Can we figure out the quality of our air?
13.) Can we figure out how fresh and how nutritious our food is?
14.) Can we easily figure out what is in our food?
15.) Can we find out how safe our water is?
16.) Can we easily make recycled paper.
17.) What are the most effective ways to use less water?
18.) How can we lower cost and improve efficiency of solar panels.?
19.) What are the most effective ways to reduce our carbon footprint?
20.) How can we make life less susceptible to disease and climate change.
List of Questions in Science that I have
1.) How did bacteria evolve into humans?
2.) How advanced will the Human race become?
3.) Will we ever lived in a society with artificial intelligence?
4.) Can we stop global warming?
5.) Will we be able to manipulate what genes we pass on to our children?
6.) Can we find an effective cure for viral infections?
7.) Can we make medicine with minimal side effects?
8.) Can we travel to other galaxies?
9.) Can we find a source of energy that is cheap, reliable, renewable, and non-polluting?
10.) Can we have vaccines against cancer?
11.) Can we map an evolutionary tree that maps relationships between all species that have ever existed.
12.) Can we figure out the quality of our air?
13.) Can we figure out how fresh and how nutritious our food is?
14.) Can we easily figure out what is in our food?
15.) Can we find out how safe our water is?
16.) Can we easily make recycled paper.
17.) What are the most effective ways to use less water?
18.) How can we lower cost and improve efficiency of solar panels.?
19.) What are the most effective ways to reduce our carbon footprint?
20.) How can we make life less susceptible to disease and climate change.