In this unit, I learned about the molecules that make up life. I first learned about the basics of chemistry. This included the parts of an atom and the properties of water. Atoms are made up of protons, with a positive charge, neutrons with a neutral charge, and electrons with a negative charge. One of the properties of water is that it is polar. This means that the oxygen atom has more of the electrons than the hydrogen atoms. This allows water to form hydrogen bonds. Water is also very cohesive, meaning that it likes to stick to other water molecules, and it is very adhesive, meaning that it likes to stick with other substances. We also learned about the pH scale, which ranks substances on a scale of 1-14 based on how much h+ ions they have. Having less h+ ions, a ranking from 1-6, means that the substance is acidic. Having more h+ ions, a ranking from 8-14, means that the substance is basic. Water is neutral, with a score of 7.
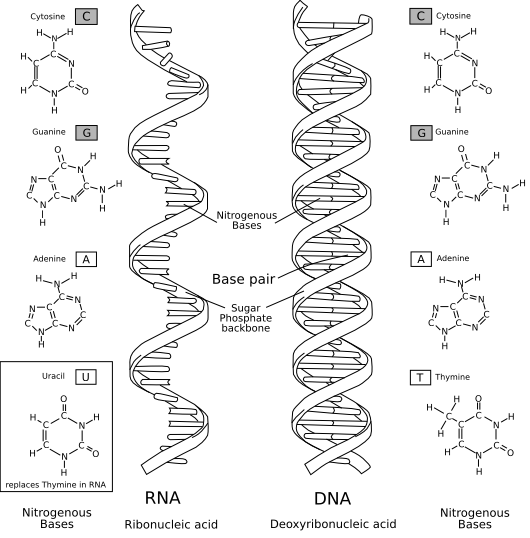
Next, I learned about the 4 macromolecules: carbohydrates, lipids, nucleic acids, and proteins. Carbohydrates are made up of carbon, hydrogen, and oxygen. They form ring like structures. Monosaccharides have one ring, disaccharides have two rings, and polysaccharides have many rings. The function of carbohydrates is to store energy. Lipids are made up of hydrogen and carbon. They are usually hydrophobic. Their function is to store energy and make up membranes. Most membranes are made up of phospholipid membranes. The inside is hydrophobic, while the outside is hydrophillic. Lipids are put into 2 classes: unsaturated fats and saturated fats. Saturated fats are just straight chains, but unsaturated fats are double bonded. nucleic acids are made up of neucleotides. Nucleic acids are made up of a base, a sugar, and a phosphate group. They are classified into 2 groups: DNA and RNA, and they contain genetic material that makes you "you." Finally, proteins are mad up of amino acids. Proteins do many jobs, such as speeding up reactions and providing structure.
Lastly, I learned about enzymes and what affects them. Enzymes help speed up reactions by lowering the activation energy. Enzymes can be affected by pH and temperature. When conditions are not optimal, the enzyme denatures.
 |
| Lipid |
 |
| Carbon |
 |
| Nucleic acid |



No comments:
Post a Comment